7.1.5: sp³ Hybrid Orbitals and the Structure of Ethane
After completing this section, you should be able to describe the structure of ethane in terms of the sp3 hybridization of the two carbon atoms present in the molecule ethane.
Bonding in Ethane
The simplest molecule with a carbon-carbon bond is ethane, C2H6.
Representations of Ethane
In ethane (CH3CH3), both carbons are sp3-hybridized, meaning that both have four bonds with tetrahedral geometry. An sp3 orbital of one carbon atom overlaps end to end with an sp3 orbital of the second carbon atom to form a carbon-carbon σ bond. This orbital overlap is often described using the notation: sp3(C)-sp3(C). Each of the remaining sp3 hybrid orbitals overlaps with the s orbital of a hydrogen atom to form carbon–hydrogen σ bonds.
The σ carbon-carbon bond has a bond length of 154 pm, and a bond strength of 377 kJ/mol. The carbon-hydrogen σ bonds are slightly weaker, 421 kJ/mol, than those of methane. The C-C-H bond angles in ethane are 111.2o which is close to the what is expected for tetrahedral molecules.
The orientation of the two CH3 groups is not fixed relative to each other. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. This means, in the case of ethane molecule, that the two methyl (CH3) groups can be pictured as two wheels on a hub, each one able to rotate freely with respect to the other. In Section 3.7 we will learn more about the implications of rotational freedom in sigma bonds, when we discuss the ‘conformation’ of organic molecules
The tetrahedral geometry of carbon was predicted as far back as 1874. But how did they know? A question came up when looking at ethane with a bromine substituent (C2H5Br). When looking at the possible structures of the compound C2H5Br there are several possible structural formulas.here was a serious problem as to whether these formulas represent the same or different compounds. All that was known in the early days was that every purified sample of C2H5Br, no matter how prepared, had a boiling point of 38 oC and density of 1.460 gml−1. Furthermore, all looked the same, all smelled the same, and all underwent the same chemical reactions. There was no evidence that C2H5Br was a mixture or that more than one compound of this formula could be prepared. One might conclude, therefore, that all of the structural formulas above represent a single substance but how? A brilliant solution to the problem came when J. H. van ‘t Hoff proposed that all four bonds of carbon are equivalent and directed to the corners of a regular tetrahedron. If we redraw the structures for C2H5Br with both carbons having tetrahedral geometry, we see that there is only one possible arrangement. This theory hints at the idea of free rotation around sigma bonds which will be discussed later.
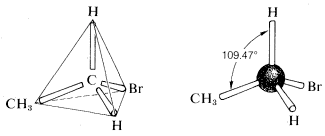
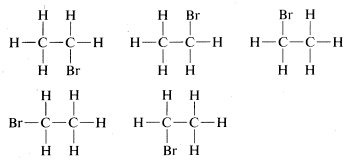
There was a serious problem as to whether these formulas represent the same or different compounds. All that was known in the early days was that every purified sample of C2H5Br, no matter how prepared, had a boiling point of 38 oC and density of 1.460 gml−1. Furthermore, all looked the same, all smelled the same, and all underwent the same chemical reactions. There was no evidence that C2H5Br was a mixture or that more than one compound of this formula could be prepared. One might conclude, therefore, that all of the structural formulas above represent a single substance but how? A brilliant solution to the problem came when J. H. van ‘t Hoff proposed that all four bonds of carbon are equivalent and directed to the corners of a regular tetrahedron. If we redraw the structures for C2H5Br with both carbons having tetrahedral geometry, we see that there is only one possible arrangement. This theory hints at the idea of free rotation around sigma bonds which will be discussed later.

Pop Quiz

