7.1.8: Hybridization of Nitrogen, Oxygen, Phosphorus and Sulfur
After completing this section, you should be able to apply the concept of hybridization of atoms such as N, O, P and S to explain the structures of simple species containing these atoms.
Make certain that you can define, and use in context, the key term below.
- lone pair electrons
Nitrogen is frequently found in organic compounds. As with carbon atoms, nitrogen atoms can be sp3-, sp2– or sp‑hybridized.
Note that, in this course, the term “lone pair” is used to describe an unshared pair of electrons.
The valence-bond concept of orbital hybridization can be extrapolated to other atoms including nitrogen, oxygen, phosphorus, and sulfur. In other compounds, covalent bonds that are formed can be described using hybrid orbitals.
Nitrogen
Bonding in NH3
The nitrogen in NH3 has five valence electrons. After hybridization these five electrons are placed in the four equivalent sp3 hybrid orbitals. The electron configuration of nitrogen now has one sp3 hybrid orbital completely filled with two electrons and three sp3 hybrid orbitals with one unpaired electron each. The two electrons in the filled sp3 hybrid orbital are considered non-bonding because they are already paired. These electrons will be represented as a lone pair on the structure of NH3. The three unpaired electrons in the hybrid orbitals are considered bonding and will overlap with the s orbitals in hydrogen to form N-H sigma bonds. Note! This bonding configuration was predicted by the Lewis structure of NH3.
The four sp3 hybrid orbitals of nitrogen orientate themselves to form a tetrahedral geometry. The three N-H sigma bonds of NH3 are formed by sp3(N)-1s(H) orbital overlap. The fourth sp3 hybrid orbital contains the two electrons of the lone pair and is not directly involved in bonding.

Methyl amine
The nitrogen is sp3 hybridized which means that it has four sp3 hybrid orbitals. Two of the sp3 hybridized orbitals overlap with s orbitals from hydrogens to form the two N-H sigma bonds. One of the sp3 hybridized orbitals overlap with an sp3 hybridized orbital from carbon to form the C-N sigma bond. The lone pair electrons on the nitrogen are contained in the last sp3 hybridized orbital. Due to the sp3 hybridization the nitrogen has a tetrahedral geometry. However, the H-N-H and H-N-C bonds angles are less than the typical 109.5o due to compression by the lone pair electrons.
Oxygen
Bonding in H2O
The oxygen in H2O has six valence electrons. After hybridization these six electrons are placed in the four equivalent sp3 hybrid orbitals. The electron configuration of oxygen now has two sp3 hybrid orbitals completely filled with two electrons and two sp3 hybrid orbitals with one unpaired electron each. The filled sp3 hybrid orbitals are considered non-bonding because they are already paired. These electrons will be represented as a two sets of lone pair on the structure of H2O . The two unpaired electrons in the hybrid orbitals are considered bonding and will overlap with the s orbitals in hydrogen to form O-H sigma bonds. Note! This bonding configuration was predicted by the Lewis structure of H2O.
The four sp3 hybrid orbitals of oxygen orientate themselves to form a tetrahedral geometry. The two O-H sigma bonds of H2O are formed by sp3(O)-1s(H) orbital overlap. The two remaining sp3 hybrid orbitals each contain two electrons in the form of a lone pair.
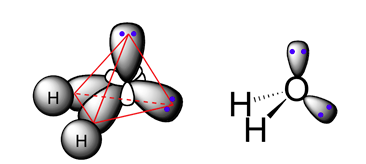
Methanol
The oxygen is sp3 hybridized which means that it has four sp3 hybrid orbitals. One of the sp3 hybridized orbitals overlap with s orbitals from a hydrogen to form the O-H sigma bonds. One of the sp3 hybridized orbitals overlap with an sp3 hybridized orbital from carbon to form the C-O sigma bond. Both the sets of lone pair electrons on the oxygen are contained in the remaining sp3 hybridized orbital. Due to the sp3 hybridization the oxygen has a tetrahedral geometry. However, the H-O-C bond angles are less than the typical 109.5o due to compression by the lone pair electrons.
Phosphorus
Methyl phosphate
The bond pattern of phosphorus is analogous to nitrogen because they are both in period 15. However, phosphorus can have have expanded octets because it is in the n = 3 row. Typically, phosphorus forms five covalent bonds. In biological molecules, phosphorus is usually found in organophosphates. Organophosphates are made up of a phosphorus atom bonded to four oxygens, with one of the oxygens also bonded to a carbon. In methyl phosphate, the phosphorus is sp3 hybridized and the O-P-O bond angle varies from 110° to 112o.
Sulfur
Methanethiol & Dimethyl Sulfide
Sulfur has a bonding pattern similar to oxygen because they are both in period 16 of the periodic table. Because sulfur is positioned in the third row of the periodic table it has the ability to form an expanded octet and the ability to form more than the typical number of covalent bonds. In biological system, sulfur is typically found in molecules called thiols or sulfides. In a thiol, the sulfur atom is bonded to one hydrogen and one carbon and is analogous to an alcohol O-H bond. In a sulfide, the sulfur is bonded to two carbons. The simplest example of a thiol is methane thiol (CH3SH) and the simplest example of a sulfide is dimethyl sulfide [(CH3)3S]. In both cases the sulfur is sp3 hybridized, however the sulfur bond angles are much less than the typical tetrahedral 109.5o being 96.6o and 99.1o respectively.
methanethiol
dimethyl sulfide
Exercises
1) Insert the missing lone pairs of electrons in the following molecules, and tell what hybridization you expect for each of the indicated atoms.
a) The oxygen is dimethyl ether:
b) The nitrogen in dimethyl amine:
c) The phosphorus in phosphine:
d) The sulfur in hydrogen sulfide:
Solutions
1)
a) sp3 hybridization
b) sp3 hybridization
c) sp3 hybridization
d) sp3 hybridization
1.10: Hybridization of Nitrogen, Oxygen, Phosphorus and Sulfur
1.10 Exercises
Questions
Q1.10.1
Identify geometry and lone pairs on each heteroatom of the molecules given.
Solutions
S1.10.1
Diethyl ether would have two lone pairs of electrons and would have a bent geometry around the oxygen.
Dimethyl amine would have one lone pair and would show a pyramidal geometry around the nitrogen.
Find the word

