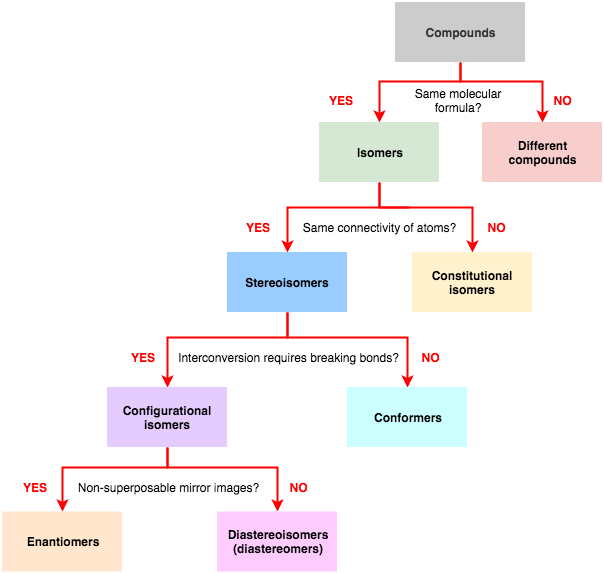
8.1: Types of Isomers
After completing this section, you should be able to explain the differences among constitutional (structural) isomers and stereoisomers (geometric isomers).
Make certain that you can define, and use in context, the key terms below.
- constitutional
- (structural) isomers
- stereoisomers
The following flow chart can be used to identify the relationship of two compounds with respect to isomerization:
Conformational Isomers
The C–C single bonds in ethane, propane, and other alkanes are formed by the overlap of an sp3 hybrid orbital on one carbon atom with an sp3 hybrid orbital on another carbon atom, forming a σ bond. Each sp3 hybrid orbital is cylindrically symmetrical (all cross-sections are circles), resulting in a carbon–carbon single bond that is also cylindrically symmetrical about the C–C axis. Because rotation about the carbon–carbon single bond can occur without changing the overlap of the sp3 hybrid orbitals, there is no significant electronic energy barrier to rotation. Consequently, many different arrangements of the atoms are possible, each corresponding to different degrees of rotation. Differences in three-dimensional structure resulting from rotation about a σ bond are called differences in conformation, and each different arrangement is called a conformational isomer (or conformer).
Conformational Isomers of Pentane
Structural Isomers
Unlike conformational isomers, which do not differ in connectivity, structural isomers differ in connectivity, as illustrated here for 1-propanol and 2-propanol. Although these two alcohols have the same molecular formula (C3H8O), the position of the –OH group differs, which leads to differences in their physical and chemical properties.
In the conversion of one structural isomer to another, at least one bond must be broken and reformed at a different position in the molecule. Consider, for example, the following five structures represented by the formula C5H12:
Of these structures, (a) and (d) represent the same compound, as do (b) and (c). No bonds have been broken and reformed; the molecules are simply rotated about a 180° vertical axis. Only three—n-pentane (a) and (d), 2-methylbutane (b) and (c), and 2,2-dimethylpropane (e)—are structural isomers. Because no bonds are broken in going from (a) to (d) or from (b) to (c), these alternative representations are not structural isomers. The three structural isomers—either (a) or (d), either (b) or (c), and (e)—have distinct physical and chemical properties.
Stereoisomers
Stereoisomers have the same connectivity in their atoms but a different arrangement in three-dimensional space. There are different classifications of stereoisomers depending on how the arrangements differ from one another. Notice that in the structural isomers, there was some difference in the connection of atoms. For example, 1-butene has a double bond followed by two single bonds while 2-butene has a single bond, then a double bond, then a single bond. A stereoisomer will have the same connectivity among all atoms in the molecule.
Geometric Isomers
With a molecule such as 2-butene, a different type of isomerism called geometric isomerism can be observed. Geometric isomers are isomers in which the order of atom bonding is the same but the arrangement of atoms in space is different. The double bond in an alkene is not free to rotate because of the nature of the bond. Therefore, there are two different ways to construct the 2-butene molecule (see figure below). The image below shows the two geometric isomers, called cis-2-butene and trans-2-butene.
Geometric Isomers of 2-Butene
The cis isomer has the two single hydrogen atoms on the same side of the molecule, while the trans isomer has them on opposite sides of the molecule. In both molecules, the bonding order of the atoms is the same. In order for geometric isomers to exist, there must be a rigid structure in the molecule to prevent free rotation around a bond. This occurs with a double bond or a ring. In addition, the two carbon atoms must each have two different groups attached in order for there to be geometric isomers. Propene (see figure below) has no geometric isomers because one of the carbon atoms (the one on the far left) involved in the double bond has two single hydrogens bonded to it.
Physical and chemical properties of geometric isomers are generally different. As with alkenes, alkynes display structural isomerism beginning with 1-butyne and 2-butyne. However, there are no geometric isomers with alkynes because there is only one other group bonded to the carbon atoms that are involved in the triple bond.
Optical Isomers
Stereoisomers that are not geometric isomers are known as optical isomers. Optical isomers differ in the placement of substituted groups around one or more atoms of the molecule. They were given their name because of their interactions with plane-polarized light. Optical isomers are labeled enantiomers or diastereomers.
Enantiomers are non-superimposable mirror images. A common example of a pair of enantiomers is your hands. Your hands are mirror images of one another but no matter how you turn, twist, or rotate your hands, they are not superimposable.
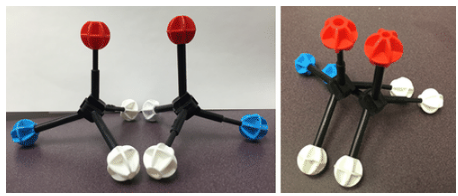
Two models that are mirror images and superimposable.
Since they are superimposable, they are the same molecule and are not isomers.
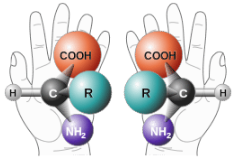
Your hands and some molecules are mirror images but are not superimposable.
These pairs of molecules are called enantiomers.
Objects that have non-superimposable mirror images are called chiral. When examining a molecule, carbon atoms with four unique groups attached are considered chiral. Look at the figure below to see an example of a chiral molecule. Note that we have to look beyond the first atom attached to the central carbon atom. The four circles indicate the four unique groups attached to the central carbon atom, which is chiral.
A chiral carbon has four unique groups attached to it
Another type of optical isomer are diastereomers, which are non-mirror image optical isomers. Diastereomers have a different arrangement around one or more atoms while some of the atoms have the same arrangement. As shown in the figure below, note that the orientation of groups on the first and third carbons are different but the second one remains the same so they are not the same molecule. The solid wedge indicates a group coming out of the page/screen towards you and the dashed line indicates that a group is going away from you “behind” the page/screen.
Diastereomers differ at one or more atom. These molecules are not mirror images and they are not superimposable.
They are optical isomers because they have the same connectivity between atoms but a different arrangement of substituent groups.
Epimers are a sub-group of diastereomers that differ at only one location. All epimers are diastereomers but not all diastereomers are epimers.
Epimers have a different arrangement around one atom, while arrangements around the other atoms are the same
Diagram showing the division of stereoisomers (also known as optical isomers)
What kind of isomers are the following pairs?
- Answer
-
- Since both structures have the same formula (C6H12) and they have the same connectivity, but are in a different arrangement of the atoms in space, they are conformational isomers.
- Since both structures have the same formula (C2H6O) but different connectivity, they are structural isomers.
- Since both structures have the same formula (C3H9Br) and the same connectivity but the structure on the left has R stereochemistry and the structure on the right has S stereochemistry, they are stereoisomers called enantiomers.
What kind of isomers are the following pairs?
- Answer
-
a) Since both structures have the same formula (C2H6O) but different connectivity of where the double bond is, they are structural isomers.
b) Both structures have the same formula and the same connectivity but the structure on the left has (R,S) stereochemistry and the structure on the right has (S,S) stereochemistry, they are stereoisomers that are diastereomers.
c) Both structures and have the same connectivity but their stereochemistry differs so they are stereoisomers. Since the one on the left is (R,S) and the one on the right is (S,R), they are non-super imposable mirror images of each other and enantiomers.
What is the relationship between the following pairs?
c) (R) – 2 chlorohexane & 1-chlorohexane
d) (2R, 3R) dichlorohexane & (2S, 3R) dichlorohexane
- Answer
-
a) Both structures have the same connectivity and same chemical formula and when you rotate the structure on the right you can see that these are the same compound both with (R,S) stereochemistry.
b) ) Since both structures have the same formula (C4H8) and the same connectivity they are stereoisomers since they differ in what is known as cis/trans isomerism (covered more in Ch 7).Due to the pi bond between carbons 2 & 3, there is not free rotation between the C2-C3 bond so the structure on the left has the carbon chains on the same side of the double bond (cis) and the structure on the right has the carbon chains on opposite sides of the double bond (trans).
c) Looking at the structures below, you can see that they have the same formula (C6H13Cl) and different connectivity, so they are structural isomers.
d) Looking at the structures below, you can see that they have the same formula (C6H12Br2) and the same connectivity, but the structure on the left is (R,R) and the structure on the right is (S,R) so since they differ by only one stereocenter, they are stereoisomers that are diastereomers (& epimers in this case).
Pop Quiz

