7.1.2: Development of Chemical Bonding Theory
After completing this section, you should be able to
- draw Lewis Dot Symbols for main group elements and ions.
- describe the three-dimensional nature of molecules.
- sketch a tetrahedral molecule, CX4, using the “wedge-and-broken-line” method of representation.
- make a ball-and-stick model of a simple tetrahedral molecule such as methane, CH4.
- draw Lewis Dot Structures for 2 electron group molecules.
- draw Lewis Dot Structures for 3 electron group molecules.
- draw Lewis Dot Structures for 4 electron group molecules.
Make certain that you can define, and use in context, the key terms below.
- bond strength
- covalent bond
- ionic bond
- Lewis structure
- lone-pair electron
- non-bonding electron
Study Notes
To draw Lewis structures successfully, you need to know the number of valence electrons present in each of the atoms involved. Memorize the number of valence electrons possessed by each of the elements commonly encountered in organic chemistry: C, H, O, N, S, P and the halogens.
When drawing any organic structure, you must remember that a neutral carbon atom will almost always have four bonds. Similarly, hydrogen always has one bond; neutral oxygen atoms have two bonds; and neutral nitrogen atoms have three bonds. By committing these simple rules to memory, you can avoid making unnecessary mistakes later in the course.
The “wedge-and-broken-line” type of representation, which helps to convey the three-dimensional nature of organic compounds, will be used throughout the course.
Bonding Overview
Why are some substances chemically bonded molecules and others are an association of ions? The answer to this question depends upon the electronic structures of the atoms and nature of the chemical forces within the compounds. Although there are no sharply defined boundaries, chemical bonds are typically classified into three main types: ionic bonds, covalent bonds, and metallic bonds. In this chapter, each type of bond and the general properties found in typical substances in which the bond type occurs will be discussed.
- Ionic bonds results from electrostatic forces that exist between ions of opposite charge. These bonds typically involve a metal with a nonmetal
- Covalent bonds result from the sharing of electrons between two atoms. The bonds typically involve one nonmetallic element with another
- Metallic bonds are found in solid metals (copper, iron, aluminum) with each metal atom bonded to several neighboring metal atoms and the bonding electrons are free to move throughout the 3-dimensional structure.
Each bond classification is discussed in detail in subsequent sections of the chapter. Let’s look at the preferred arrangements of electrons in atoms when they form chemical compounds.
Lewis Symbols
At the beginning of the 20th century, the American chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbols, often shortened to Lewis dot symbols—that can be used for predicting the number of bonds formed by most elements in their compounds. Each Lewis dot symbol consists of the chemical symbol for an element surrounded by dots that represent its valence electrons.
- provide a convenient representation of valence electrons
- allows you to keep track of valence electrons during bond formation
- consists of the chemical symbol for the element plus a dot for each valence electron
To write an element’s Lewis dot symbol, we place dots representing its valence electrons, one at a time, around the element’s chemical symbol. Up to four dots are placed above, below, to the left, and to the right of the symbol (in any order, as long as elements with four or fewer valence electrons have no more than one dot in each position). The next dots, for elements with more than four valence electrons, are again distributed one at a time, each paired with one of the first four. Fluorine, for example, with the electron configuration [He]2s22p5, has seven valence electrons, so its Lewis dot symbol is constructed as follows:
![]()

Lewis used the unpaired dots to predict the number of bonds that an element will form in a compound. Consider the symbol for nitrogen in Figure 7.1.2.2. The Lewis dot symbol explains why nitrogen, with three unpaired valence electrons, tends to form compounds in which it shares the unpaired electrons to form three bonds. Boron, which also has three unpaired valence electrons in its Lewis dot symbol, also tends to form compounds with three bonds, whereas carbon, with four unpaired valence electrons in its Lewis dot symbol, tends to share all of its unpaired valence electrons by forming compounds in which it has four bonds. Lewis symbols are a tool to help draw structures. We will see why bonding in molecular compounds follow Lewis’ theory in the next section.
Elements in the same group have the same number of valence electrons and similar Lewis symbols. For example, the electron configuration for atomic sulfur is [Ne]3s23p4, thus there are six valence electrons. Its Lewis symbol would therefore be similar to oxygen and look like:
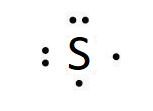
The Octet Rule
Lewis’s major contribution to bonding theory was to recognize that atoms tend to lose, gain, or share electrons to reach a total of eight valence electrons, called an octet. This so-called octet rule explains the stoichiometry of most compounds in the s and p blocks of the periodic table. We now know from quantum mechanics that the number eight corresponds to one ns and three np valence orbitals, which together can accommodate a total of eight electrons. Remarkably, though, Lewis’s insight was made nearly a decade before Rutherford proposed the nuclear model of the atom. Common exceptions to the octet rule are helium, whose 1s2 electron configuration gives it a full n = 1 shell, and hydrogen, which tends to gain or share its one electron to achieve the electron configuration of helium.
Lewis’s idea of an octet explains why noble gases rarely form compounds. They have the stable s2p6 configuration (full octet, no charge), so they have no reason to react and change their configuration. All other elements attempt to gain, lose, or share electrons to achieve a noble gas configuration. This explains why atom combine together to form compounds. By forming bond the makes the atoms more stable and lower in energy. Making bonds releases energy and represents a driving force for the formation of compounds.
Atoms often gain, lose, or share electrons to achieve the same number of electrons as the noble gas closest to them in the periodic table.
Lewis Structures
Lewis structures represent how Lewis symbols gain, lose, or share electrons to obtain an octet by forming compounds.
Lewis Structures of Ionic Compounds
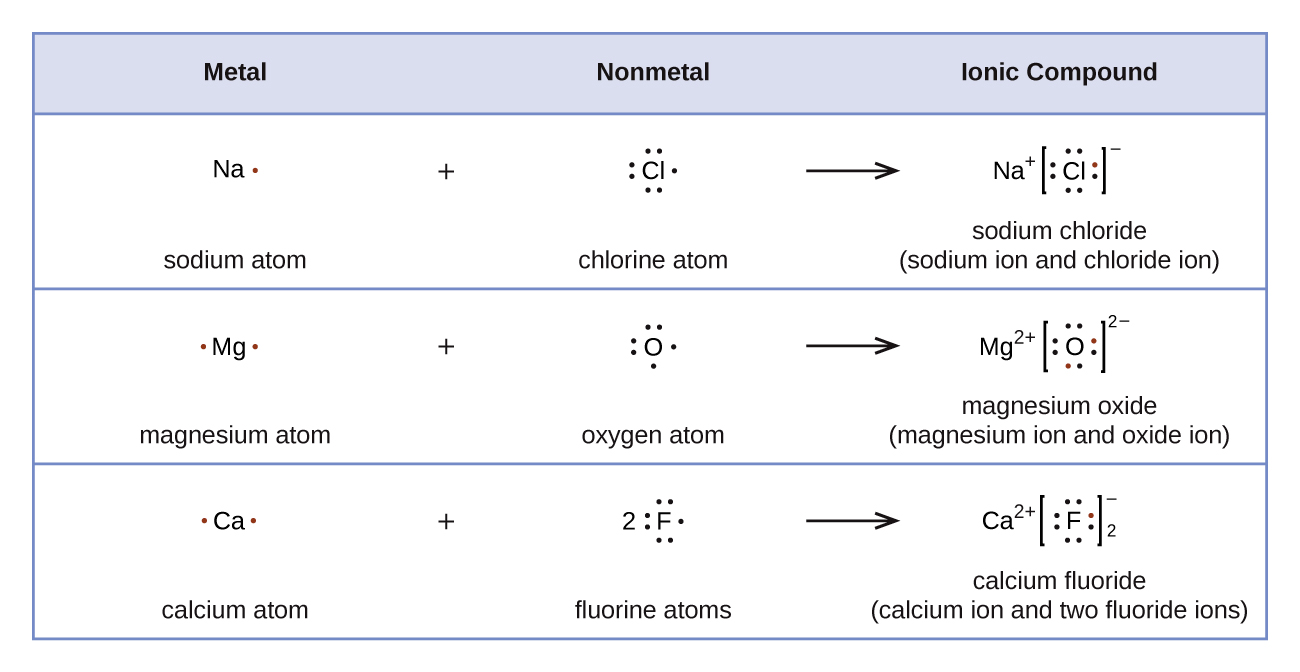
Whenever there is a metal present in the structure of an organic compound, there is a high likelihood that at least one ionic bond is present. Ionic bonds are represented differently in Lewis structures than covalent bonds. Great care should be taken whenever drawing the Lewis structure of an organic compounds which contains and ionic bond. Ionic bonds typically are formed when a metal and a nonmetal are part of a compound. Some atoms achieve an octet by fully gaining or losing electrons to form ions. Ionic bonds form through the eletrostatic attraction of the created ions. The formula for table salt is NaCl. It is the result of Na+ ions and Cl– ions bonding together. If sodium metal and chlorine gas mix under the right conditions, they will form salt. The sodium loses an electron, and the chlorine gains that electron. In the process, a great amount of light and heat is released. The resulting salt is mostly unreactive — it is stable. It will not undergo any explosive reactions, unlike the sodium and chlorine that it is made of. Why? Referring to the octet rule, atoms attempt to get a noble gas electron configuration, which is eight valence electrons. Sodium (1s22s22p63s1) has one valence electron, so giving it up would result in the same electron configuration as neon (1s22s22p6). Chlorine (1s22s22p63s23p7)has seven valence electrons, so if it takes one it will have eight (an octet). Chlorine has the electron configuration of argon (1s22s22p63s23p8) when it gains an electron.
The Lewis structure of an ionic compound show the movement of electrons. For NaCl, sodium is in group 1 and has one valence electron and chlorine is in group 17 and has seven valence electrons. Sodium loses its sole valence electron thereby becomes positively charged. Chlorine gains this electron, gaining a full octet, and a negative charge. After the gain/loss of an electron the new Lewis structures of Na+ and Cl– are written next to each other representing the ionic bond in NaCl.
Examples of Lewis Structures of Ionic Compounds
Covalent Bonds and the Lewis Structures of Molecular Compounds
While alkali metals (such as sodium and potassium), alkaline earth metals (such as magnesium and calcium), and halogens (such as fluorine and chlorine) often form ions in order to achieve a full octet, the principle elements of organic chemistry – carbon, hydrogen, nitrogen, and oxygen – instead tend to fill their octet by sharing electrons with other atoms, forming covalent bonds. Consider the simplest case of hydrogen gas. An isolated hydrogen atom has only one electron, located in the 1s orbital. If two hydrogen atoms come close enough so that their respective 1s orbitals overlap, the two electrons can be shared between the two nuclei, and a covalently bonded H2 molecule is formed. In the Lewis structure of H2, each pair of electrons that is shared between two atoms is drawn as a single line, designating a single covalent bond.
Hydrogen represents a special case, because a hydrogen atom cannot fulfill the octet rule; it needs only two electrons to have a full shell. This is often called the ‘doublet rule’ for hydrogen.
One of the simplest organic molecules is methane with the molecular formula CH4. Methane is the ‘natural gas’ burned in home furnaces and hot water heaters, as well as in electrical power generating plants. To illustrate the covalent bonding in methane using a Lewis structure, we first must recognize that, although a carbon atom has a total of six electrons it’s Lewis symbol has four unpaired electrons. Following Lewis’ theory the carbon atom wants to form four covalent bonds to fill its octet. In a methane molecule, the central carbon atom shares its four valence electrons with four hydrogen atoms, thus forming four bonds and fulfilling the octet rule (for the carbon) and the ‘doublet rule’ (for each of the hydrogens).
The next relatively simple organic molecule to consider is ethane, which has the molecular formula C2H6. If we draw each atom’s Lewis symbol separately, we can see that the octet/doublet rule can be fulfilled for all of them by forming one carbon-carbon bond and six carbon-hydrogen bonds.
The same approach can be used for molecules in which there is no carbon atom. In a water molecule, the Lewis symbol of the oxygen atom has two unpaired electrons. These are paired with the single electron in the Lewis symbols of the hydrogens’ two O-H covalent bonds. The remaining four non-bonding electrons on oxygen called ‘lone pairs’.
Since the lone pair electrons are often NOT shown in chemical structures, it is important to see mentally add the lone pairs. In the beginning, it can be helpful to physically add the lone pair electrons.
| methylamine | ethanol | chloromethane |
Exercise
For the following structure, please fill in all of the missing lone pair electrons.
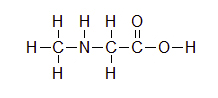
Answer
When two or more electrons are shared between atoms a multiple covalent bond is formed. The molecular formula for ethene (also known as ethylene, a compound found in fruits, such as apples, that signals them to ripen) is C2H4. Arranging Lewis symbols of the atoms, you can see that the octet/doublet rule can be fulfilled for all atoms only if the two carbons share two pairs of electrons between them. Ethene contains a carbon/carbon double bond.
Following this pattern, the triple bond in ethyne molecular formula C2H2, (also known as acetylene, the fuel used in welding torches), is formed when the two carbon atoms share three pairs of electrons between them.
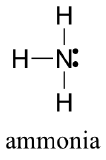
Draw the Lewis structure for ammonia, NH3.
- Answer
Molecular Shape
A stick and wedge drawing of methane shows the tetrahedral angles…(The wedge is coming out of the paper and the dashed line is going behind the paper. The solid lines are in the plane of the paper.)
The following examples make use of this notation, and also illustrate the importance of including non-bonding valence shell electron pairs when viewing such configurations.
| Methane | Ammonia | Water |
Bonding configurations are readily predicted by valence-shell electron-pair repulsion theory, commonly referred to as VSEPR in most introductory chemistry texts. This simple model is based on the fact that electrons repel each other, and that it is reasonable to expect that the bonds and non-bonding valence electron pairs associated with a given atom will prefer to be as far apart as possible. The bonding configurations of carbon are easy to remember, since there are only three categories.
| Configuration | Bonding Partners | Bond Angles | Example |
|---|---|---|---|
| Tetrahedral | 4 | 109.5º | 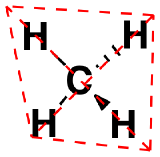 |
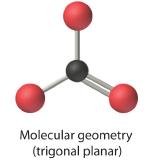
| Trigonal Planar | 3 | 120º | 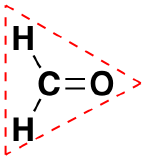 |
| Linear | 2 | 180º |  |
Figure 1.4.3
In the three examples shown above, the central atom (carbon) does not have any non-bonding valence electrons; consequently the configuration may be estimated from the number of bonding partners alone. However, for molecules of water and ammonia, the non-bonding electrons must be included in the calculation. In each case there are four regions of electron density associated with the valence shell so that a tetrahedral bond angle is expected. The measured bond angles of these compounds (H2O 104.5º & NH3 107.3º) show that they are closer to being tetrahedral than trigonal planar or linear. Of course, it is the configuration of atoms (not electrons) that defines the the shape of a molecule, and in this sense ammonia is said to be pyramidal (not tetrahedral). The compound boron trifluoride, BF3, does not have non-bonding valence electrons and the configuration of its atoms is trigonal. Nice treatments of VSEPR theory have been provided by Oxford and Purdue. The best way to study the three-dimensional shapes of molecules is by using molecular models. Many kinds of model kits are available to students and professional chemists.
Two Electron Groups
Our first example is a molecule with two bonded atoms and no lone pairs of electrons, BeH2.
AX2: BeH2
1. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. The Lewis electron structure is
2. There are two electron groups around the central atom. We see from Figure 1.4.3 that the arrangement that minimizes repulsions places the groups 180° apart.
3. Both groups around the central atom are bonding pairs (BP). Thus BeH2 is designated as AX2.
4. From Figure 1.4.3 we see that with two bonding pairs, the molecular geometry that minimizes repulsions in BeH2 is linear.
AX2: CO2
1. The central atom, carbon, contributes four valence electrons, and each oxygen atom contributes six. The Lewis electron structure is
2. The carbon atom forms two double bonds. Each double bond is a group, so there are two electron groups around the central atom. Like BeH2, the arrangement that minimizes repulsions places the groups 180° apart.
3. Once again, both groups around the central atom are bonding pairs (BP), so CO2 is designated as AX2.
4. VSEPR only recognizes groups around the central atom. Thus the lone pairs on the oxygen atoms do not influence the molecular geometry. With two bonding pairs on the central atom and no lone pairs, the molecular geometry of CO2 is linear (Figure 1.4.3).
Three Electron Groups
AX3: BCl3
1. The central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. The Lewis electron structure is
2. There are three electron groups around the central atom. To minimize repulsions, the groups are placed 120° apart (Figure 1.4.3).
3. All electron groups are bonding pairs (BP), so the structure is designated as AX3.
4. From Figure 1.4.3 we see that with three bonding pairs around the central atom, the molecular geometry of BCl3 is trigonal planar.
AX3: CO32−
1. The central atom, carbon, has four valence electrons, and each oxygen atom has six valence electrons. As you learned previously, the Lewis electron structure of one of three resonance forms is represented as
2. The structure of CO32− is a resonance hybrid. It has three identical bonds, each with a bond order of 4/3.We minimize repulsions by placing the three groups 120° apart (Figure 1.4.3).
3. All electron groups are bonding pairs (BP). With three bonding groups around the central atom, the structure is designated as AX3.
4. We see from Figure 1.4.3 that the molecular geometry of CO32− is trigonal planar.
In our next example we encounter the effects of lone pairs and multiple bonds on molecular geometry for the first time.
-
AX2E: SO2
1. The central atom, sulfur, has 6 valence electrons, as does each oxygen atom. With 18 valence electrons, the Lewis electron structure is shown below.
2. There are three electron groups around the central atom, two double bonds and one lone pair. We initially place the groups in a trigonal planar arrangement to minimize repulsions (Figure 1.4.3).
3. There are two bonding pairs and one lone pair, so the structure is designated as AX2E. This designation has a total of three electron pairs, two X and one E. Because a lone pair is not shared by two nuclei, it occupies more space near the central atom than a bonding pair (Figure 1.4.4). Thus bonding pairs and lone pairs repel each other electrostatically in the order BP–BP < LP–BP < LP–LP. In SO2, we have one BP–BP interaction and two LP–BP interactions.
4. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. Thus with two nuclei and one lone pair the shape is bent, or V shaped, which can be viewed as a trigonal planar arrangement with a missing vertex (Figures 1.4.2.1 and 1.4.3).
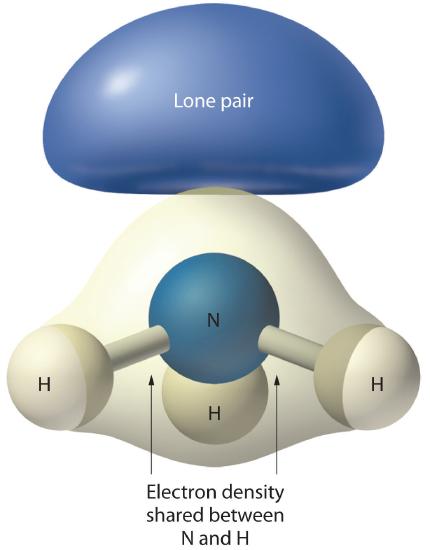
Figure 1.4.4: The Difference in the Space Occupied by a Lone Pair of Electrons and by a Bonding Pair As with SO2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom.
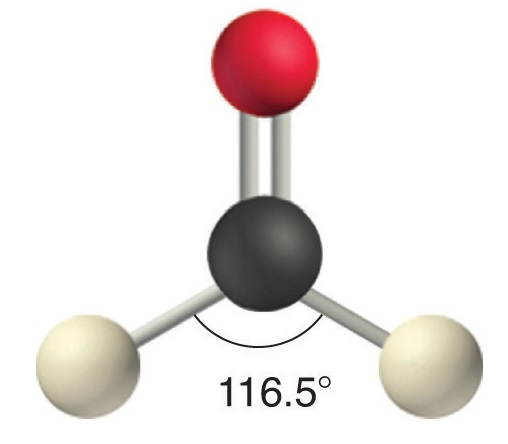
Like lone pairs of electrons, multiple bonds occupy more space around the central atom than a single bond, which can cause other bond angles to be somewhat smaller than expected. This is because a multiple bond has a higher electron density than a single bond, so its electrons occupy more space than those of a single bond. For example, in a molecule such as CH2O (AX3), whose structure is shown below, the double bond repels the single bonds more strongly than the single bonds repel each other. This causes a deviation from ideal geometry (an H–C–H bond angle of 116.5° rather than 120°).
Four Electron Groups
One of the limitations of Lewis structures is that they depict molecules and ions in only two dimensions. With four electron groups, we must learn to show molecules and ions in three dimensions.
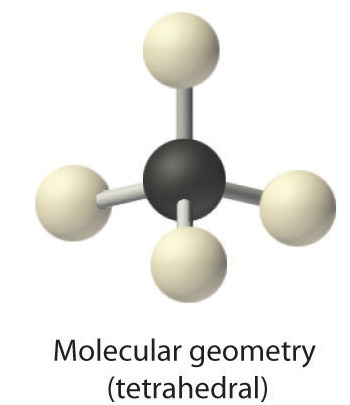
AX4: CH4
1. The central atom, carbon, contributes four valence electrons, and each hydrogen atom has one valence electron, so the full Lewis electron structure is
2. There are four electron groups around the central atom. As shown in Figure 1.4.2, repulsions are minimized by placing the groups in the corners of a tetrahedron with bond angles of 109.5°.
3. All electron groups are bonding pairs, so the structure is designated as AX4.
4. With four bonding pairs, the molecular geometry of methane is tetrahedral (Figure 1.4.3).
AX3E: NH3
1. In ammonia, the central atom, nitrogen, has five valence electrons and each hydrogen donates one valence electron, producing the Lewis electron structure
2. There are four electron groups around nitrogen, three bonding pairs and one lone pair. Repulsions are minimized by directing each hydrogen atom and the lone pair to the corners of a tetrahedron.
3. With three bonding pairs and one lone pair, the structure is designated as AX3E. This designation has a total of four electron pairs, three X and one E. We expect the LP–BP interactions to cause the bonding pair angles to deviate significantly from the angles of a perfect tetrahedron.
4. There are three nuclei and one lone pair, so the molecular geometry is trigonal pyramidal. In essence, this is a tetrahedron with a vertex missing (Figure 1.4.3). However, the H–N–H bond angles are less than the ideal angle of 109.5° because of LP–BP repulsions.
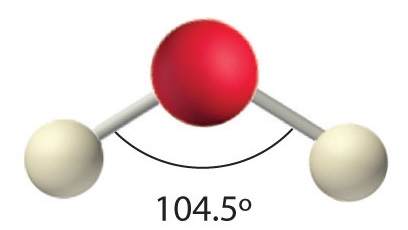
AX2E2: H2O
1. Oxygen has six valence electrons and each hydrogen has one valence electron, producing the Lewis electron structure
2. There are four groups around the central oxygen atom, two bonding pairs and two lone pairs. Repulsions are minimized by directing the bonding pairs and the lone pairs to the corners of a tetrahedron Figure 1.4.3.
3. With two bonding pairs and two lone pairs, the structure is designated as AX2E2 with a total of four electron pairs. Due to LP–LP, LP–BP, and BP–BP interactions, we expect a significant deviation from idealized tetrahedral angles.
4. With two hydrogen atoms and two lone pairs of electrons, the structure has significant lone pair interactions. There are two nuclei about the central atom, so the molecular shape is bent, or V shaped, with an H–O–H angle that is even less than the H–N–H angles in NH3, as we would expect because of the presence of two lone pairs of electrons on the central atom rather than one.. This molecular shape is essentially a tetrahedron with two missing vertices.
Pop Quiz

