7.1.4: sp³ Hybrid Orbitals and the Structure of Methane
After completing this section, you should be able to describe the structure of methane in terms of the sp3 hybridization of the central carbon atom.
Make certain that you can define, and use in context, the key terms below.
- bond angle
- hybridization
- sp3 hybrid
The tetrahedral shape is a very important one in organic chemistry, as it is the basic shape of all compounds in which a carbon atom is bonded to four other atoms. Note that the tetrahedral bond angle of [latex]\ce{\sf{H−C−H}}[/latex] is 109.5°.
Valence Bond Theory
Valence bond theory’s use of overlapping atomic orbitals to explain how chemical bonds form works well in simple diatomic molecules such as H2. However, when molecules with more than two atoms form stable bonds, we require a more detailed model. A good example is methane (CH4). According to valence bond theory, the structure of a covalent species can be depicted using a Lewis structure.
Experimentally, it has been shown that the four carbon-hydrogen bonds in the methane molecule are identical, meaning they have the same bond energy and the same bond length. Also, VSEPR theory suggests that the geometry at the carbon atom in the methane molecule is tetrahedral (2), and there exists a large body of both theoretical and experimental evidence supporting this prediction.
According to valence bond theory, to form a covalent bond forms when an unpaired electron in one atom overlaps with an unpaired electron in a different atom. Now, consider the the electron configuration of the four valence electrons in carbon.
There is a serious mismatch between the electron configuration of carbon (1s22s22p2) and the predicted structure of methane. The modern structure shows that there are only 2 unpaired electrons to share with hydrogens, instead of the 4 needed to create methane. Also, the px and py orbitals are at 90o to each other. They would form perpendicular bonds instead of the tetrahedral 109.5o bond angle predicted by VSEPR and experimental data. Lastly, there are two different orbitals, 2s and 2p, which would create different types of C-H bonds. As noted earlier, experimentally, the four carbon-hydrogen bonds in the methane molecule are identical.
Hybrid Orbitals
An answer to the problems posed above was offered in 1931 by Linus Pauling. He showed mathematically that an s orbital and three p orbitals on an atom can combine to form four equivalent hybrid atomic orbitals.
- Hybrid orbitals do not exist in isolated atoms. They are formed only in covalently bonded atoms.
- Hybrid orbitals have shapes and orientations that are very different from those of the atomic orbitals in isolated atoms.
- A set of hybrid orbitals is generated by combining atomic orbitals. The number of hybrid orbitals in a set is equal to the number of atomic orbitals that were combined to produce the set.
- All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
- The type of hybrid orbitals formed in a bonded atom create the molecular geometry as predicted by the VSEPR theory.
- Hybrid orbitals overlap to form σ bonds.
- Lone pair electrons are often contained in hybrid orbitals
sp3 Hybridization in Methane
In order to explain this observation, valence bond theory relies on a concept called orbital hybridization. In this picture, the four valence orbitals of the carbon (one 2s and three 2p orbitals) combine mathematically (remember: orbitals are described by equations) to form four equivalent hybrid orbitals, which are named sp3 orbitals because they are formed from mixing one s and three p orbitals. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons.
The shape of an sp3 hybridized orbital is a combination of s and p atomic orbitals.
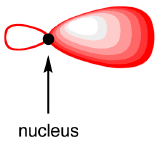
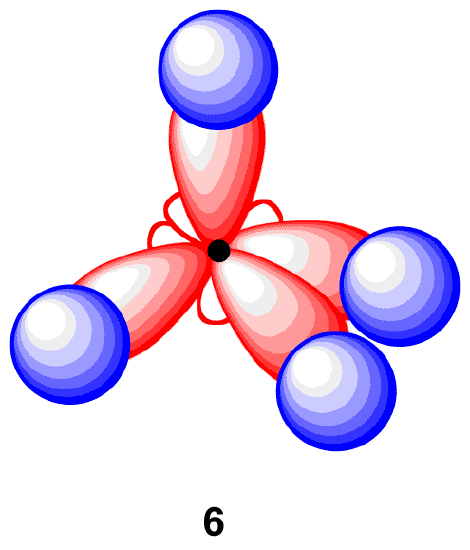
Each sp3-hybridized orbital bears an electron, and electrons repel each other. To minimize the repulsion between electrons, the four sp3-hybridized orbitals arrange themselves around the carbon nucleus so that they are as far away as possible from each other, resulting in the tetrahedral arrangement predicted by VSPER. The carbon atom in methane is called an “sp3-hybridized carbon atom.” The larger lobes of the sp3 hybrids are directed towards the four corners of a tetrahedron, meaning that the angle between any two orbitals is 109.5o.

Bonding in Methane
Each C-H bond in methane, then, can be described as an overlap between a half-filled 1s orbital in four hydrogen atoms and the larger lobe of one of the four half-filled sp3 hybrid orbitals form a four equivalent sigma (σ) bond. This orbital overlap is often described using the notation: sp3(C)-1s(H). The formation of sp3 hybrid orbitals successfully explains the tetrahedral structure of methane and the equivalency of the the four C-H bonds.
What remains is an explanation of why the sp3 hybrid orbitals form. When the s and 3 p orbitals in carbon hybridize the resulting sp3 hybrid orbital is unsymmetrical with one lobe larger than the other. This means the larger lobe can overlap more effectively with orbitals from other bonds making them stronger. Hybridizing allows for the carbon to form stronger bonds than it would with unhybridized s or p orbitals.
The four carbon-hydrogen bonds in methane are equivalent and all have a bond length of 109 pm (1.09 x 10-10 m), bond strength of of 429 kJ/mol. All of the H-C-H bond angles are 109.5o.
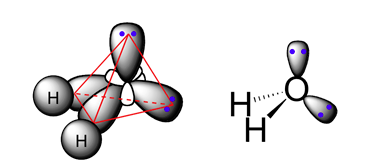
Arguably the most influential chemist of the 20th century, Linus Pauling (1901–1994) is the only person to have won two individual (that is, unshared) Nobel Prizes. In the 1930s, Pauling used new mathematical theories to enunciate some fundamental principles of the chemical bond. His 1939 book The Nature of the Chemical Bond is one of the most significant books ever published in chemistry.
Pauling’s big contribution to chemistry was valence bond theory, which combined his knowledge of quantum mechanical theory with his knowledge of basic chemical facts, like bond lengths and and bond strengths and shapes of molecules. Valence bond theory, like Lewis’s bonding theory, provides a simple model that is useful for predicting and understanding the structures of molecules, especially for organic chemistry. .
By 1935, Pauling’s interest turned to biological molecules, and he was awarded the 1954 Nobel Prize in Chemistry for his work on protein structure. (He was very close to discovering the double helix structure of DNA when James Watson and James Crick announced their own discovery of its structure in 1953.) He was later awarded the 1962 Nobel Peace Prize for his efforts to ban the testing of nuclear weapons.

Linus Pauling was one of the most influential chemists of the 20th century.
In his later years, Pauling became convinced that large doses of vitamin C would prevent disease, including the common cold. Most clinical research failed to show a connection, but Pauling continued to take large doses daily. He died in 1994, having spent a lifetime establishing a scientific legacy that few will ever equal
Find the word

